Quality Manual
MS, NMOSD, MOGAD
Recommendations for healthcare professionals on the diagnosis and treatment of MS, NMOSD and MOGAD, including patient informed consent forms
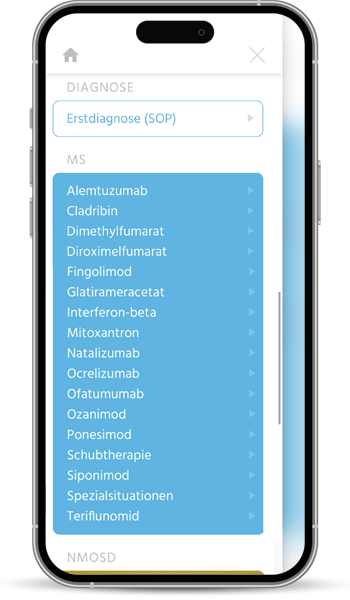
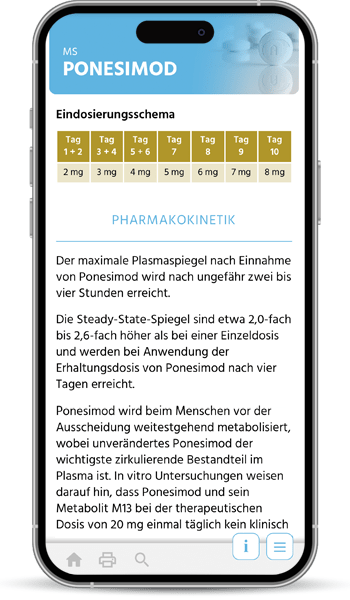
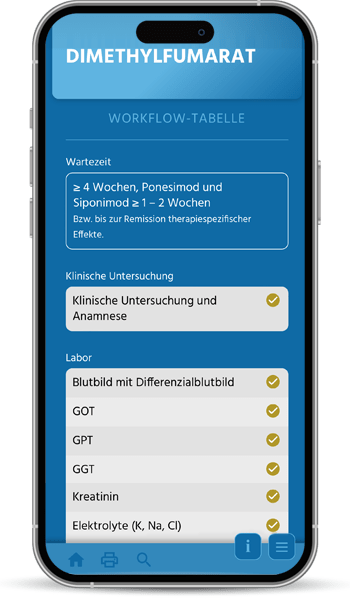
The first quality manuals for the diagnosis and treatment of multiple sclerosis were developed by the KKNMS in 2012, when the approval of new drugs heralded a development that continues to this day: Drug therapy for MS is becoming more diverse, better, but also potentially riskier. In particular, changes in therapy pose an increasing challenge in terms of effectiveness and the avoidance of side effects.
Our quality manual is moving with the times and is now available as a user-friendly online application with optimal access on all devices to our independent and practical information on MS, NMOSD and MOGAD therapy.
The handbook and the associated patient informed consent forms are written by experienced practitioners and scientists, regularly updated and carefully coordinated in our committees. We recommend it to all treating neurologists.